Current Programs
The Diabetic Foot Consortium (DFC), funded by the National Institutes of Health is the first-ever multicenter network to study diabetic foot ulcers, a common and burdensome complication of diabetes and the leading cause of lower limb amputations in the United States. The DFC aims to lay the foundation for a clinical trial network to test how to improve diabetic wound healing and prevent amputations among the 27 million American adults with diabetes. The DFC is supported by the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
The first studies will focus on finding biological clues, called biomarkers, in people with diabetic foot ulcers that can guide treatment and predict how the ulcer will heal and the likelihood of an ulcer returning. For example, the first study of the DFC, led by Indiana University School of Medicine, will test whether body fluid leaking through the skin on a newly healed ulcer can predict how likely an ulcer might return. A second study, led by the University of Miami, will test whether the presence of or a change in specific cellular proteins in tissue samples from an ulcer can predict the likelihood of healing in the next 12 weeks. Up to 34% of people with diabetes will develop a foot ulcer in their lifetime and half of foot ulcers become infected. Each year about 100,000 Americans with diabetes will lose part of their lower limb because a foot ulcer becomes infected or does not heal.
The NIDDK, a component of the NIH, conducts and supports research on diabetes and other endocrine and metabolic diseases; digestive diseases, nutrition and obesity; and kidney, urologic and hematologic diseases. Spanning the full spectrum of medicine and afflicting people of all ages and ethnic groups, these diseases encompass some of the most common, severe and disabling conditions affecting Americans. For more information about the NIDDK and the NIH, visit http://www.niddk.nih.gov and https://www.nih.gov.
For more information, please contact Brent Toto.
View the website here.
Under the leadership of regenerative medicine expert Chandan Sen, PhD, a non-invasive, nanochip device using a technology called tissue nanotransfection (TNT) has been developed to reprogram one type of tissue into another with a simple touch and an electric field harmless to the body. The technology has two major components: First, a nanotechnology-based chip hardware is designed to deliver cargo to adult cells in the live body. Second, the design of specific biological cargo for cell conversion allows the cargo, when delivered using nanotechnology-based chip hardware, to convert an adult cell from one type to another.
Tissue nanotransfection is non-invasive, does not require any laboratory-based procedures, and may be implemented at the point of care. In less than a second, the nanochip can deliver treatment at the injury site and convert skills to vasculogenic cells.
This technology avoids the use of stem cells and is simple to use. Tissue nanotransfection has been licensed with the ultimate goal of enabling skin and other tissue to be converted to tissue types necessary for therapy. These potential therapies include healing burns, reducing diabetic complications, treating injured soldiers, and re-growing damaged and diseased tissue.
Dr. Sen and his team of interdisciplinary experts continue to develop and innovate this first-of-its-kind technology at the McGowan Institute for Regenerative Medicine.
Perspective Videos on tissue nanotransfection technology
For more information, please contact Brent Toto.
In the United States, among patients with diabetes, 15 percent develop a foot ulcer, and 12 to 24 percent of individuals with a foot ulcer require amputation. With funding (1U01DK119099-01) from the National Institutes of Health, the McGowan Institute for Regenerative Medicine at the University of Pittsburgh School of Medicine is able to treat diabetic foot ulcer with leading expertise.
The institute is equipped with an interdisciplinary research and education program that delivers cutting-edge innovation that leads to the development of a Clinical Research Unit (CRU). The institute is the major participant in this multi-site research consortium, focused on propelling new scientific discoveries to be expeditiously translated to better care for patients with diabetic foot ulcers.
The institute is focused on providing wound-healing expertise and wound care of patient populations with diabetic foot ulcers. Using an efficient clinical and scientific infrastructure, the center is developing evidentiary criteria for qualifying/validating the targeted diabetic foot ulcer biomarker. Research faculty are developing and implementing novel strategies to monitor and address the confounding factors relevant to biomarker study outcomes. The center will establish Clinical Research Unit administration and achieve Diabetic Foot Consortium (DFC) readiness.
UPMC Wound Healing Services
The McGowan Institute for Regenerative Medicine works with the UPMC Wound Healing Services in treating complicated and non-healing wounds, using the most current, advanced wound dressings and leading-edge wound-care technologies to provide the best possible outcome. Learn more about our research and facilities.
Burn and Biofilm Infection
When the barrier function of the skin is breached during burn wound, microbial infection colonizes the injured area. Microbial infestation leads to compromised host response to injury. The Centers for Disease Control and Prevention and National Institutes of Health estimate that more than 65 percent of all human infections caused by microbes exhibit a biofilm phenotype.
Pathogenic biofilms are microbial communities within self-secreted extracellular polymeric substance (EPS) collectively made up of carbohydrates, microbial proteins and DNA along with host substances. Such physical protective barrier confer antibiotic tolerance to microbes, thereby giving benefit to biofilm over planktonic form. This gives biofilms a more resistant version of infection and generates a potential candidate for antimicrobial strategies.
At the McGowan Institute for Regenerative Medicine, research efforts are focused on targeting molecular pathways to retard biofilm-inducible pathological mechanisms.
For more information, please contact Brent Toto.
A new generation of thought leaders seek to better understand what happens to human tissues after human death and want to trace how and why certain cells live on after human death. Discoveries may usher in new reasoning about life and death, both from a scientific and philosophical perspective, and introduce possibilities for future medical treatment. We hypothesize withdrawal of central survival signals in clinical death induces a return to fetal development in select cellular populations that strive purposefully to survive.
In 2021, Dr. Chandan Sen and his team received a three-year grant from the John Templeton Foundation on human post-mortem tissue conatus research that helps address one of the foundation’s strategic priorities: Science and the Big Questions. Led by Chandan K. Sen, PhD, the McGowan Institute for Regenerative Medicine will focus on the skin, which has the capacity to live at a cellular level long after death. Research findings could help inform future biomedical research that recognizes death as a biological variable as well as a pathway to future clinical care that supports enhanced tissue survival that could have broad clinical and investigative implications, including transplant and forensic science. Faculty from University of Pittsburgh and our collaborators at Indiana University College of Arts and Sciences, with expertise in philosophy and bioethics, are collaborating with McGowan investigators to study the biophyscial, ethical and philosophical questions that will direct their efforts in discovery.
For more information, please contact Brent Toto.
View the website here.
Critical Limb Ischemia (CLI) is a peripheral artery disease caused due to massive occlusion of blood flow to lower extremities. It is often associated with excruciating pain and leads to the development of skin ulcers or gangrene.
The five-year survival rate of Critical Limb Ischemia patients is 50 percent after initial diagnosis. For patients that fall into the extreme spectrum of Critical Limb Ischemia that cannot tolerate surgical intervention, limb amputation is the only treatment option. Cell-based therapies provide immense hope to promote wound healing and restore blood perfusion in treating Critical Limb Ischemia patients.
In recent clinical trials, McGowan colleagues at Indiana University School of Medicine have successfully achieved blood perfusion restoration using intramuscular injection of endometrial regenerative cells (ERC) (NCT01558908) and autologous bone marrow aspirate (NCT01049919) in Critical Limb Ischemia patients. Based on these advancements, the McGowan Institute for Regenerative Medicine is engaged in collaborative efforts from basic scientists, bioengineers and vascular surgeons to develop novel cell-based therapies for Critical Limb Ischemia and related diseases.
For more information, please contact Brent Toto.
With advanced battlefield technologies, field medicine and ever-changing arenas of battle, military personnel may face limb loss and other complex issues, but many have returned and active in our communities after serving. The primary goal of this program is to help military personnel lead normal, productive lives and perform daily living tasks that are comparable to people without limb loss. An important secondary goal is to enable the return of affected personnel to active-duty status. At the McGowan Institute for Regenerative Medicine, scientists and clinicians work with government organizations and industry to address pressing military and veteran needs.
Military Population Focused Solutions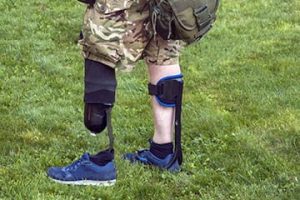
Military servicemembers have distinctly different prosthetic needs. Our research considers the unique anatomical characteristics of the individual to develop solutions that are specifically suited for them. Aesthetics is an important consideration in this approach. The goal is to maintain and improve residual limb health across a lifetime, coupled with the need to push boundaries in terms of durability, functionality and performance.
Limb Health
Residual limb health is central to the well-being and physical ability of the amputee. Current patient-based research at McGowan employs in-socket diagnostics and imaging to assess limb health. Observations lead to novel mechanistic hypotheses, which in turn inform clinical study design. The program can monitor and manage limb volume, limb temperature and other vital health outcomes, which significantly inform advancements in prosthetic technology, resections and performance outcomes.
Academic, Industry and Public Partnerships
Our research solutions are driven by the end user, collaborator feedback and health needs. Solutions should have solid commercialization strategies with defined timelines and outcomes. Optimal industry partnerships help us innovate and reach new heights in improving the health and daily living. For example, a past project focused on further developing an adaptive socket system that detects in-socket residual limb motion and dynamically adjusts internal socket negative pressure to optimize fit and performance. This VA-funded project developed a product which went to market two years after the inception of the idea and is currently used by veterans.
Emerging Regenerative Rehabilitation of Improved Prothesis Control
An amputation can be a traumatic event that can leave affected patients feeling unsure of their capacity to thrive. Advances in upper limb prosthetic technologies with the innovative approach of Targeted Muscle Transfer (TMR) and Targeted Sensory Re-innervation (TSR) allow patients to move and feel their prosthetics without thought. Residual nerves from the amputated limb are transferred to reinnervate new muscle targets that have otherwise lost function. These reinnervated muscles then serve as biological amplifiers of the amputated nerve motor signals, enabling more intuitive control of advanced prosthetic arms.
This new surgical technique will overcome challenges associated with current approaches such as lack of precise control and lack of coordinated motions. It allows the amputee to use more than one pair of muscles intuitively and receive feedback sensation from their prostheses. Ajay K. Seth, MD, a board-certified orthopedic hand and upper extremity surgeon and orthopedic researcher, performed the first Targeted Muscle Re-Innervation and Targeted Sensory Re-Innervation surgery on a transhumeral amputee, an amputation through the arm. The surgery was successful and is now a living example of the newly emerging technology of amputee research where patients can sense through their prosthetics. Dr. Seth is closely working with Mohamed S. El Masry, MBBCH, under the guidance of Chandan K. Sen, PhD, and Sashwati Roy, PhD, to develop a pre-clinical program aimed at understanding the limits of TMR and TSR.
For more information, please contact Brent Toto.
Tissue engineering encompass the generation of surrogate functional tissue using biomaterials. Over the last decade, this field of medical science has become an important alternative of restoring functional integrity of damaged tissue.
The 3D Stem Cell Biology Research Group at the University of Pittsburgh School of Medicine pioneered the technique of differentiating pluripotent human stem cells into inner ear organoid. This regenerative approach helped in promoting sensory hair-cell regeneration to restore hearing loss.
The program also investigates generation of intact skin from stem cells. The artificial skin generated through this approach have all the critical skin components (hair follicles, sweat glands and pigmentation cells) to impart total functionality of skin graft used in reconstructive surgeries.
For more information, please contact Brent Toto.
 The Center for Military Medicine Research (CMMR), Health Sciences, is focused on applications of regenerative medicine, reconstructive medicine, transplantation immunology and neuroscience, including traumatic brain injury and neuroprosthetics, with the aim of getting innovative therapies to wounded warriors. CMMR represents a formal mechanism through which the challenges and opportunities of casualty care and wound healing can be examined at an advanced research level. It enables a network of successful partnerships and collaborations between scientists, clinicians, industry and the U.S. departments of Defense and Veterans Affairs to foster the most promising research technologies and therapeutic strategies.
The Center for Military Medicine Research (CMMR), Health Sciences, is focused on applications of regenerative medicine, reconstructive medicine, transplantation immunology and neuroscience, including traumatic brain injury and neuroprosthetics, with the aim of getting innovative therapies to wounded warriors. CMMR represents a formal mechanism through which the challenges and opportunities of casualty care and wound healing can be examined at an advanced research level. It enables a network of successful partnerships and collaborations between scientists, clinicians, industry and the U.S. departments of Defense and Veterans Affairs to foster the most promising research technologies and therapeutic strategies.
To learn more please visit www.cmmr.pitt.edu.
Dental, Oral and Craniofacial Research – Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center

Reconstruction of dental, oral, and craniofacial (DOC) tissues through tissue engineering and regenerative medicine (RM) is a major goal in dentistry. Despite the continuing success and commercial viability of RM approaches in selected areas of medicine, the application of RM concepts to DOC problems has not been well-implemented in clinical practice. To meet this need, the Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center (MPWRM RC) was established as an interdisciplinary partnership amongst the University of Michigan, the University of Pittsburgh, and the Wyss Institute, along with technology, clinical, regulatory, marketing, and commercial translation experts from private practice and industry. Through this collaborative effort, we will accelerate clinical adoption of promising technologies by providing the interdisciplinary expertise and resources to guide technologies towards FDA submissions. McGowan Director, Dr. William R. Wagner is a co-PI along with affiliated faculty member Dr. Charles Sfeir.
For more information, please reach out to Patrick Cantini at cantinip@upmc.edu.
Learn more about MPWRM RC at https://mpwrm-doctrc.pitt.edu/.
Past Programs
The National Science Foundation funded the Engineering Research Center for Revolutionizing Metallic Biomaterials (ERC-RMB) in 2008 to create resorbable biomedical devices and investigate the fundamental science of novel, resorbable materials. This includes material design and achieving improved functional outcomes.
ERC-RMB is a partnership between North Carolina A&T State University, the University of Pittsburgh and the University of Cincinnati, together with other global and international partners from industry, academia, state and local governments. Researchers across ERC-RMB capitalize on the expertise resident at each partner institution to advance both science and medical practice in the field of resorbable metallic biomedical implants.
Developing implantable medical devices made from resorbable metal is not a new idea. ERC-RMB is creating new alloys, processing and testing techniques that address clinical challenges related to resorbable metals to suit clinical demands. The consortium seeks to design devices that can adapt to changes in a patient’s body and dissolve once healing has occurred, eliminating the need for follow up procedures and potential complications of major orthopedic, craniofacial, cardiovascular and thoracic interventions. To learn more please visit http://erc.ncat.edu/.
The Pennsylvania Pediatric Medical Device Consortium (formerly the Philadelphia Pediatric Medical Device Consortium), connects Children’s Hospital of Philadelphia (CHOP) with the McGowan Institute for Regenerative Medicine based at the University of Pittsburgh. This partnership comes on the heels of a five-year, $5 million grant renewal from the Consortium’s sponsor, the U.S. Food and Drug Administration. The mission of the PPDC is to support the development and commercialization of promising medical devices that address unmet clinical needs in children.
The PMD Launchpad, the home for the PPDC’s resources and services, represents the collaborative efforts of the PPDC with the FDA’s Pediatric Device Consortia program that has sites located across the United States. Leveraging our collective expertise in the fields of pediatrics, medical device development, and biomedical engineering, our goal is to advance the development and accessibility of medical devices for the pediatric patient population. Below are the PPDC members who actively play an integral role in making the PMD Launchpad an important source of information and resources to address your interests in the pediatric medical device field.
For more information, contact Patrick Cantini at pmc20@pitt.edu or Jamelle Price at jap260@pitt.edu
With research in low earth orbit (LEO) becoming more accessible, the McGowan Institute convened over 150 thought leaders from around the country, during the height of the pandemic, to review the current scientific research about the effects of microgravity on organs, tissues, cells, metallics, etc. The 2020 Biomanufacturing in Space Symposium reviewed space based regenerative medicine research and discussed leveraging LEO to advance biomanufacturing for regenerative medicine applications. The symposium identified areas where financial investments could stimulate advancements overcoming technical barriers. Opportunities in disease modeling, stem-cell-derived products, and biofabrication were highlighted. Over the last decade, the International Space Station National Laboratory (ISS National Lab) has supported space-based studies in the areas of tissue engineering and regenerative medicine. This initial research and development have provided important insights into how microgravity can be leveraged to advance biomanufacturing in space to benefit human life and commercial enterprise on Earth. Microgravity induces changes in bodily systems that result in effects including cardiovascular deconditioning, skeletal muscle atrophy, bone loss, and immune dysfunction, among others. Utilizing microgravity has contributed to the collective fundamental knowledge of cellular behavior, cell-cell interactions, tissue development and regeneration, and aggregate interactions in the context of a whole organism.
For more information, please contact Patrick Cantini at pmc20@pitt.edu.
The Alliance for Regenerative Rehabilitation Research and Training (AR3T), an NIH-funded resource center, is a collaboration between the University of Pittsburgh, Stanford University, Mayo Clinic, and the University of Texas at Austin. The overarching goal of AR3T is to support the expansion of scientific knowledge, expertise and methodologies across the fields of regenerative medicine and rehabilitation through education, training, research support, and funding opportunities. Regenerative Rehabilitation is an innovative field combining discoveries in tissue engineering and cellular therapies with rehabilitative protocols in order to improve treatment outcomes following injury, disease and disability.
Learn more at www.ar3t.pitt.edu.
OUR RESEARCH
FEATURED NEWS
School of Pharmacy student meet with McGowan faculty
The McGowan Institute For Regenerative Medicine2024-04-12T13:45:09+00:00April 12, 2024|
The McGowan Institute Welcomes a New Finance Team Member
The McGowan Institute For Regenerative Medicine2024-02-19T14:15:16+00:00February 19, 2024|
Dr. Singh Receives a Department of Defense Award!
The McGowan Institute For Regenerative Medicine2024-02-08T14:10:36+00:00February 8, 2024|